Exploring the molecular polarity of CO2 is a cornerstone in the study of chemistry, offering insights into how carbon dioxide behaves in diverse settings. Regardless of whether you are a student delving into chemistry or a professional working within the field, the concept of molecular polarity is indispensable. This article seeks to provide a thorough breakdown of CO2's molecular polarity, simplifying intricate ideas into accessible knowledge.
Carbon dioxide (CO2) ranks among the most prevalent molecules on Earth, playing a pivotal role in atmospheric balance, biological mechanisms, and industrial operations. Yet, its molecular framework and polarity are often misinterpreted. This article will dissect CO2's molecular structure, clarifying why it is categorized as nonpolar and shedding light on its properties.
Upon finishing this article, you will possess a holistic comprehension of CO2's molecular polarity, its ramifications, and its practical applications in real-world scenarios. Let's embark on this enlightening journey!
Contents Overview
- Understanding Molecular Polarity
- Exploring the Molecular Structure of CO2
- Defining Molecular Polarity
- Why CO2 is Classified as Nonpolar
- Examining Bond Polarity in CO2
- Dipole Moment in CO2
- Practical Applications of CO2
- Comparing CO2 with Other Molecules
- Scientific Evidence Supporting CO2 Nonpolarity
- Final Thoughts
Understanding Molecular Polarity
Molecular polarity pertains to the dispersion of electrical charges within a molecule, a concept that is fundamental to comprehending chemical interactions, solubility, and intermolecular forces. In the context of CO2, its molecular structure and symmetry significantly influence its polarity.
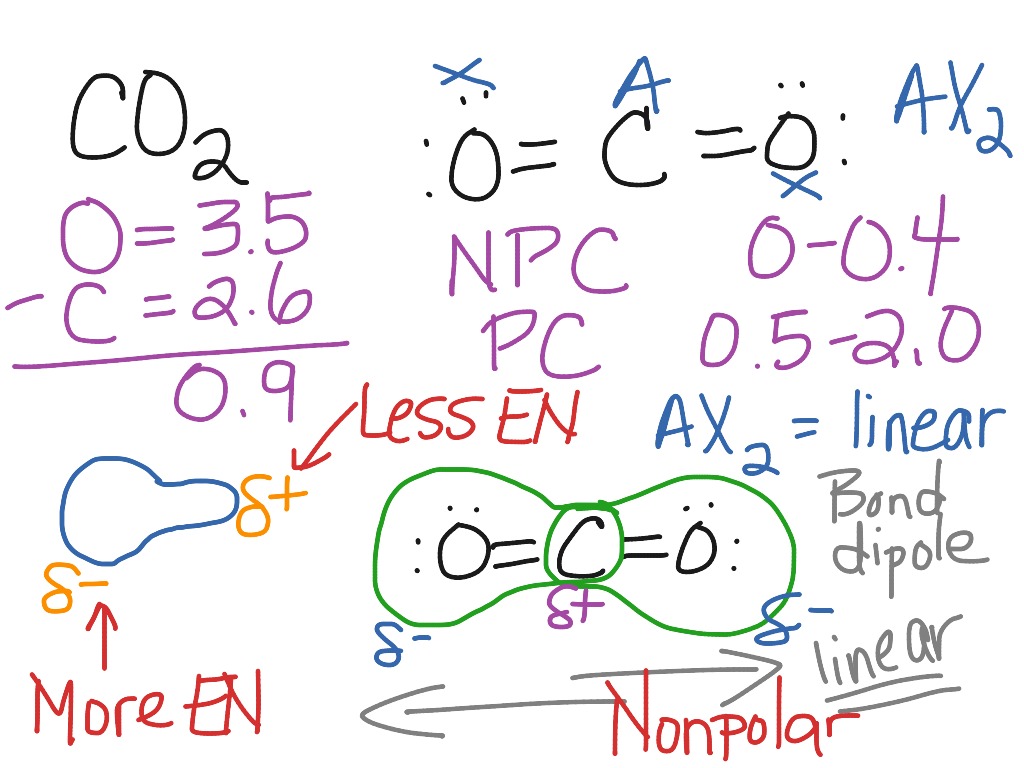
CO2 is a linear molecule, featuring a single carbon atom doubly bonded to two oxygen atoms. Despite containing polar bonds, CO2 is deemed nonpolar due to its symmetrical arrangement. This segment will delve into the reasons for this classification and its broader implications.
Exploring the Molecular Structure of CO2
The molecular structure of CO2 is notable for its linear geometry. The carbon atom is centrally positioned, with two oxygen atoms attached via double bonds. This linear configuration leads to a balanced distribution of charges, contributing to the nonpolar nature of CO2.
Salient Features of CO2's Structure
- A linear shape with bond angles precisely at 180 degrees.
- Double bonds linking carbon and oxygen atoms.
- An evenly distributed electron density.
Defining Molecular Polarity
Molecular polarity is determined by the existence and arrangement of partial charges within a molecule. A polar molecule exhibits an uneven distribution of charges, generating a dipole moment. Conversely, a nonpolar molecule demonstrates a balanced charge distribution, resulting in no net dipole moment.
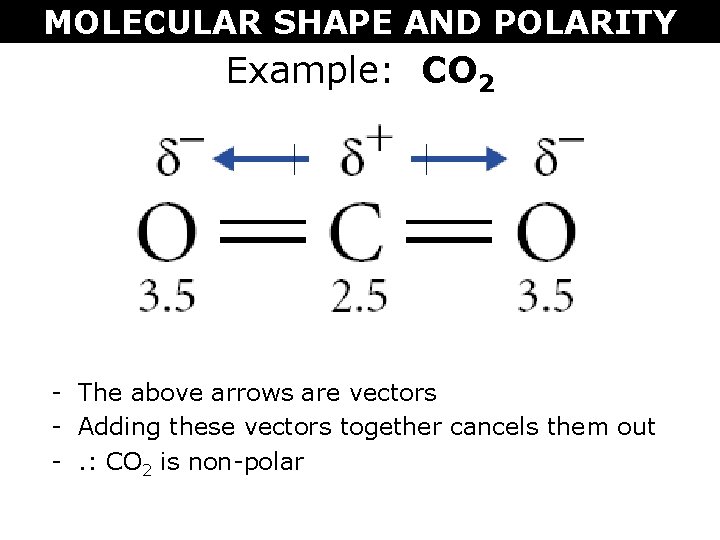
In the case of CO2, the polar C=O bonds offset each other because of the molecule's linear geometry, rendering it nonpolar. This concept will be further elaborated in subsequent sections.
Why CO2 is Classified as Nonpolar
Even though CO2 comprises polar bonds, it is identified as nonpolar due to its symmetrical configuration. The two C=O bonds in CO2 are polar since oxygen is more electronegative than carbon. However, CO2's linear geometry ensures that the dipole moments of these bonds neutralize each other, eliminating any net dipole moment.
Elements Contributing to CO2 Nonpolarity
- A linear molecular shape.
- Uniform bond lengths and angles.
- An evenly spread charge distribution.
Examining Bond Polarity in CO2
The C=O bonds in CO2 are polar due to the disparity in electronegativity between carbon and oxygen. Oxygen, being more electronegative, attracts the shared electrons closer, creating a partial negative charge (δ-) on the oxygen atoms and a partial positive charge (δ+) on the carbon atom.
Nevertheless, the symmetrical structure of the molecule ensures these partial charges balance out, resulting in an overall nonpolar molecule. This phenomenon is integral to comprehending CO2's molecular polarity.
Dipole Moment in CO2
The dipole moment gauges the separation of positive and negative charges within a molecule. In CO2, the dipole moments of the two C=O bonds are equivalent in magnitude yet opposing in direction, leading to a net dipole moment of zero.
This absence of a net dipole moment is a hallmark of nonpolar molecules, elucidating why CO2 lacks polarity despite its polar bonds.
Practical Applications of CO2
CO2's nonpolar nature has substantial implications across various disciplines, including chemistry, biology, and industry. Some of the primary applications of CO2 include:
- Carbonated Beverages: CO2 is employed to carbonate drinks, imparting the fizzy texture characteristic of sodas and sparkling water.
- Fire Extinguishers: CO2 is an effective fire suppressant because it displaces oxygen, suffocating flames.
- Industrial Processes: CO2 finds use in a multitude of industrial contexts, such as welding, refrigeration, and enhanced oil extraction.
Grasping the molecular polarity of CO2 is crucial for optimizing its utility in these applications.
Comparing CO2 with Other Molecules
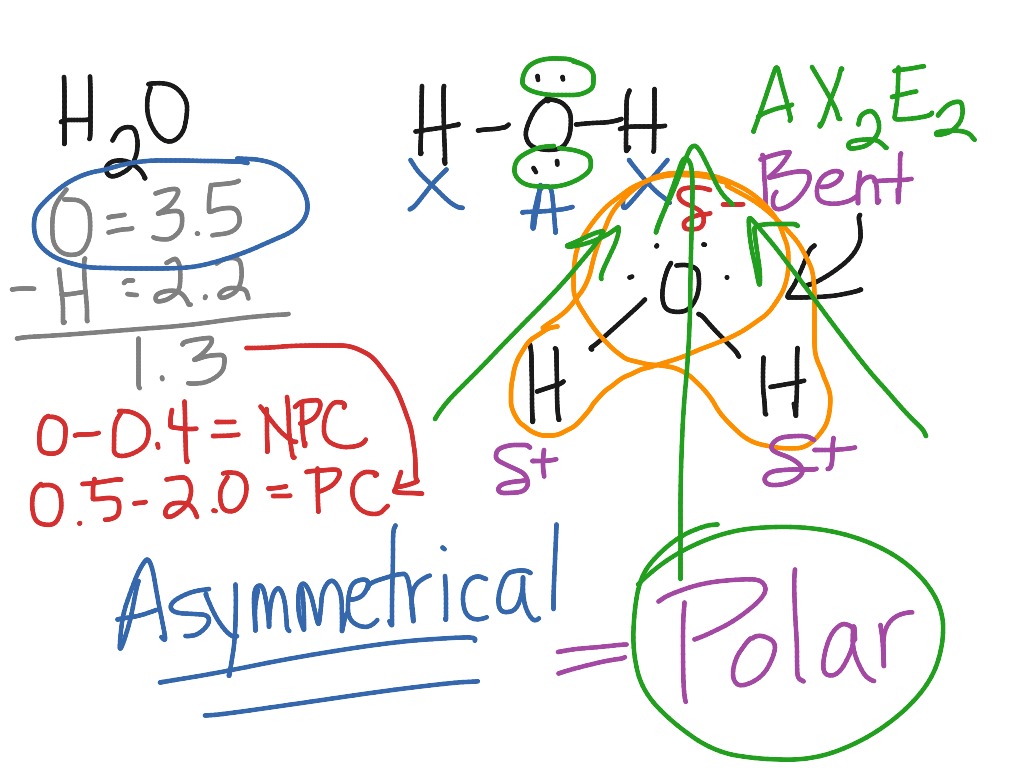
To enhance the understanding of CO2's molecular polarity, comparing it with other molecules is beneficial. For instance, water (H2O) is polar due to its bent geometry, which prevents the dipole moments of its O-H bonds from canceling out. Conversely, CO2's linear geometry ensures that its dipole moments neutralize, resulting in a nonpolar molecule.
Comparison Chart
| Molecule | Geometry | Polarity |
|---|---|---|
| CO2 | Linear | Nonpolar |
| H2O | Bent | Polar |
Scientific Evidence Supporting CO2 Nonpolarity
Empirical research has consistently validated the nonpolar nature of CO2. For example, infrared spectroscopy indicates that CO2 does not display a significant dipole moment, affirming its nonpolar classification. Moreover, CO2's behavior in different solvents aligns with the characteristics of nonpolar molecules.
These findings are corroborated by numerous studies published in reputable scientific journals, such as the Journal of Chemical Education and the American Chemical Society.
Final Thoughts
To summarize, the molecular polarity of CO2 is an intriguing subject that underscores the significance of molecular geometry in determining polarity. Despite the presence of polar bonds, CO2 is classified as nonpolar due to its symmetrical layout. This knowledge carries profound implications across various fields, from chemistry to industry.
We invite you to delve into additional resources on this topic and share your insights in the comments section below. Furthermore, feel free to explore other articles on our platform for more revelations into the realm of chemistry.
References:
- Journal of Chemical Education
- American Chemical Society
- Chemistry: The Central Science by Brown, LeMay, and Bursten
Detail Author:
- Name : Arielle Ward
- Username : flatley.fay
- Email : patricia40@weimann.com
- Birthdate : 2001-08-26
- Address : 97148 Paxton Passage Suite 691 Goyettemouth, OH 68207
- Phone : 603.457.2323
- Company : Kuhn and Sons
- Job : Aircraft Launch Specialist
- Bio : Facilis consectetur corrupti odit corrupti nobis. Minima omnis provident deserunt provident sint eum quidem incidunt. Eligendi aut deleniti debitis iure. Veniam velit delectus ut vitae ut.
Socials
linkedin:
- url : https://linkedin.com/in/skunze
- username : skunze
- bio : Aut rerum voluptatem distinctio eligendi qui.
- followers : 2679
- following : 1068
instagram:
- url : https://instagram.com/kunze1986
- username : kunze1986
- bio : Sed quidem unde sunt dolore. Mollitia ad repellat hic. Excepturi temporibus voluptatum et placeat.
- followers : 2868
- following : 832
facebook:
- url : https://facebook.com/sofia_kunze
- username : sofia_kunze
- bio : Ab eaque quidem iure. Velit molestias sint ab voluptatem sed.
- followers : 823
- following : 1443
twitter:
- url : https://twitter.com/skunze
- username : skunze
- bio : Qui quasi asperiores laborum iusto beatae occaecati. Minus nemo ipsum id rerum. Corrupti cupiditate cum et doloremque.
- followers : 1768
- following : 2230