The molecular geometry of chlorine trifluoride (CLF3) is a captivating subject in chemistry that delves into the structural arrangement of this compound. CLF3 plays a pivotal role in chemistry due to its unique characteristics and diverse applications. Exploring its geometry offers profound insights into how atoms bond and interact within molecules, which ultimately govern their physical and chemical behaviors. This article aims to provide a detailed exploration of CLF3 geometry, catering to students, researchers, and enthusiasts alike, ensuring a comprehensive understanding of this fascinating compound.
Chlorine trifluoride (CLF3) is a highly reactive compound extensively utilized in various industrial processes, including rocket propellants and etching agents. The molecular geometry of CLF3 is instrumental in determining its reactivity and functionality. By investigating the geometry of CLF3, we uncover the principles of molecular bonding and how they shape the compound's properties, making it an essential subject in the realm of chemistry.
This article is designed to offer an in-depth analysis of CLF3 geometry. It covers its structural arrangement, bond angles, hybridization, and applications. Additionally, it explores real-world applications, ensuring readers gain a thorough understanding of the subject. From its theoretical foundations to practical implications, this article provides a holistic view of CLF3 geometry.
- Who Played Lurch On Addams Family
- 60 Minutes What Is On Tonight
- Temperature For Medium Rareteak
- Latest Jeff Bridges
- South Bend A Breaking News
Table of Contents
- Introduction to CLF3 Geometry
- Molecular Structure of CLF3
- Hybridization in CLF3
- Bond Angles in CLF3
- Shape of CLF3 Molecule
- Physical and Chemical Properties
- Applications of CLF3
- Variations of CLF3 Geometry
- Comparison with Other Molecules
- Conclusion and Future Directions
Introduction to CLF3 Geometry
What is CLF3?
Chlorine trifluoride (CLF3) is a highly reactive interhalogen compound consisting of one chlorine atom bonded to three fluorine atoms. The molecular geometry of CLF3 is crucial in determining its chemical behavior. By comprehending the geometry of CLF3, chemists can predict its reactivity and stability, making it a vital subject for advanced chemistry studies. Its unique structure allows it to serve as a powerful oxidizing agent, enhancing its importance in both academic and industrial contexts.
Why Study CLF3 Geometry?
The study of CLF3 geometry holds significant value for multiple reasons. Firstly, it elucidates the compound's extraordinary reactivity and its ability to act as a potent oxidizing agent. Secondly, understanding CLF3 geometry provides critical insights into molecular bonding principles, which are foundational to the field of chemistry. Lastly, this knowledge can be applied in industries where CLF3 is employed, such as semiconductor manufacturing and aerospace engineering, ensuring safer and more efficient processes.
Molecular Structure of CLF3
The molecular structure of CLF3 is determined by the spatial arrangement of its constituent atoms. Chlorine, positioned at the center, forms covalent bonds with three fluorine atoms. Additionally, chlorine carries two lone pairs of electrons, which significantly influence the overall geometry of the molecule. This arrangement results in a unique molecular structure that contributes to CLF3's distinctive properties.
- Amc Independence Commons 20 Theater
- Outlet Centermithfield Nc
- Johnny Depp Vanessa Paradis
- Modesto Family Court
- Bogo Wings Thursday
- Central atom: Chlorine
- Bonding atoms: Three fluorine atoms
- Non-bonding electrons: Two lone pairs on chlorine
Hybridization in CLF3
What is Hybridization?
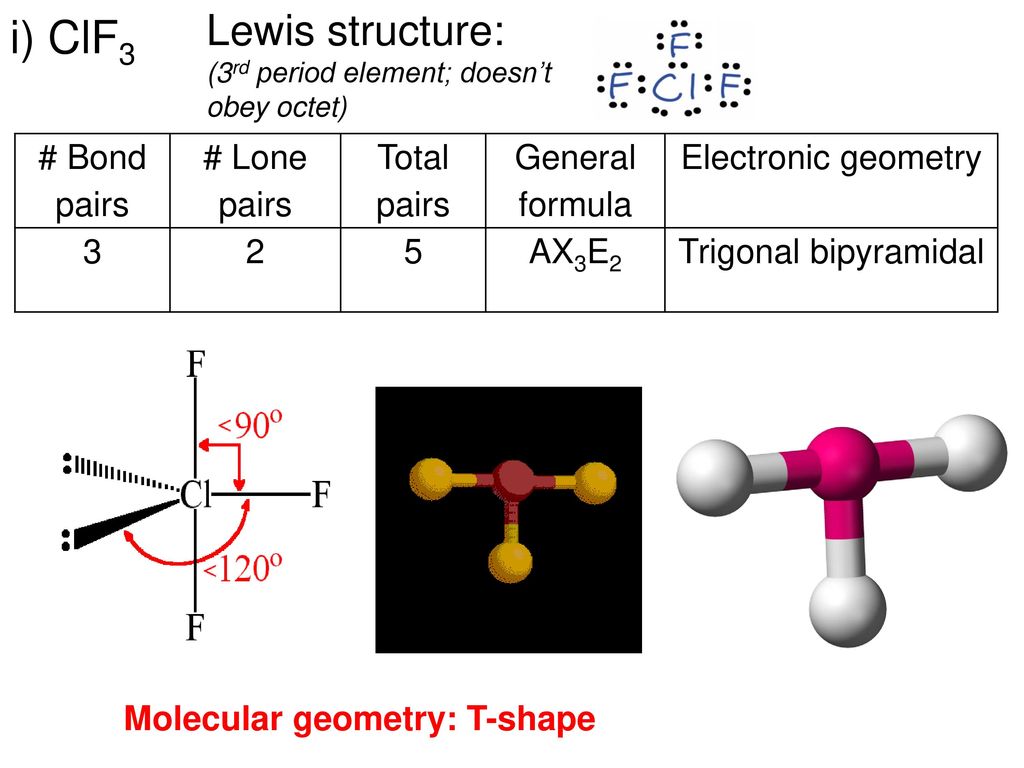
Hybridization is the process where atomic orbitals combine to form new hybrid orbitals used in bonding. In CLF3, the central chlorine atom undergoes sp3d hybridization. This hybridization produces five hybrid orbitals, with four participating in bonding with the fluorine atoms and one accommodating the lone pair of electrons. This arrangement plays a key role in determining the geometry and stability of the molecule.
Impact of Hybridization on Geometry
The sp3d hybridization in CLF3 leads to a trigonal bipyramidal electron geometry. However, due to the repulsive forces exerted by the lone pairs, the actual molecular geometry is distorted, resulting in a T-shaped structure. This distortion, explained by the VSEPR (Valence Shell Electron Pair Repulsion) theory, is a direct consequence of the lone pair-bond pair repulsion, significantly influencing the compound's chemical behavior.
Bond Angles in CLF3
The bond angles in CLF3 are influenced by the repulsion between lone pairs and bonding pairs of electrons. Ideally, in a trigonal bipyramidal arrangement, the bond angles would be 90° and 120°. However, the presence of lone pairs causes deviations from these ideal angles:
- F-Cl-F bond angles (equatorial): Approximately 87.5°
- F-Cl-F bond angles (axial): Approximately 175°
These deviations highlight the impact of lone pairs on molecular geometry, emphasizing the importance of understanding electron repulsion in predicting molecular shapes.
Shape of CLF3 Molecule
The shape of the CLF3 molecule is characterized as T-shaped. This shape arises from the trigonal bipyramidal electron geometry distorted by lone pair repulsion. The three fluorine atoms occupy the equatorial positions, while the two lone pairs occupy the axial positions. This specific arrangement minimizes electron repulsion, thereby stabilizing the molecule and influencing its reactivity.
Physical and Chemical Properties
CLF3 exhibits a range of notable physical and chemical properties:
- Appearance: Colorless gas
- Boiling Point: -8.9°C
- Melting Point: -76.3°C
- Density: 3.75 g/L (at 0°C)
- Reactivity: Highly reactive, acts as a strong oxidizing agent
These properties make CLF3 highly suitable for applications requiring strong oxidizing power, such as in the semiconductor industry and aerospace engineering.
Applications of CLF3
Industrial Uses
CLF3 finds extensive use in various industrial applications due to its high reactivity:
- Etching agent in semiconductor manufacturing
- Propellant in rocket fuels
- Oxidizing agent in chemical reactions
Environmental Considerations
Despite its utility, CLF3 poses environmental risks due to its reactivity and potential to generate harmful byproducts. Proper handling, storage, and disposal practices are essential to mitigate its environmental impact and ensure safe usage.
Variations of CLF3 Geometry
While CLF3 predominantly exhibits a T-shaped geometry, variations in its molecular geometry can occur due to external factors such as temperature, pressure, or the influence of other molecules. These variations can significantly affect the compound's reactivity and stability. Understanding these alterations is crucial for optimizing its performance in various applications and ensuring its safe and efficient use.
Comparison with Other Molecules
Similarities with Other Halides
CLF3 shares similarities with other halides, such as bromine trifluoride (BrF3). Both compounds exhibit T-shaped geometries due to the presence of lone pairs on the central atom. However, differences in atomic size and electronegativity lead to variations in bond angles and reactivity, highlighting the importance of these factors in determining molecular behavior.
Differences from Non-Halides
Compared to non-halide molecules, such as methane (CH4), CLF3's geometry is more complex due to the influence of lone pairs. Non-halide molecules typically exhibit simpler geometries due to the absence of lone pairs, underscoring the critical role of lone pairs in shaping molecular geometry and influencing chemical properties.
Conclusion and Future Directions
In conclusion, the molecular geometry of CLF3 is a fundamental aspect of understanding this highly reactive compound. Its T-shaped structure, resulting from sp3d hybridization and lone pair repulsion, significantly impacts its reactivity and applications. By studying CLF3 geometry, we gain valuable insights into molecular bonding principles and their practical implications, paving the way for advancements in chemistry and related fields.
We encourage readers to explore further topics in molecular geometry and chemistry. Share your thoughts and questions in the comments section below, and feel free to explore other articles on our website for a deeper understanding of the subject. Together, we can continue to unravel the mysteries of chemistry and contribute to its ever-evolving knowledge base.
Data sources:
- Chemistry textbooks and peer-reviewed journals
- Reputable online chemistry databases
- Industrial manuals and technical reports

Detail Author:
- Name : Jillian Roob Sr.
- Username : wferry
- Email : emery61@yahoo.com
- Birthdate : 1990-11-29
- Address : 77566 Joel Fords Lake Maudland, GA 52300-1787
- Phone : 1-629-708-4705
- Company : Kub, DuBuque and Stark
- Job : Retail Salesperson
- Bio : Animi voluptatem odio praesentium odio esse est. Ullam dolore aut in facere sit laborum molestiae. Iure vero aliquid sed est aut praesentium nobis.
Socials
tiktok:
- url : https://tiktok.com/@orval.kemmer
- username : orval.kemmer
- bio : Aliquid quaerat consectetur odit perspiciatis. Dolorem deleniti ullam qui.
- followers : 3454
- following : 694
facebook:
- url : https://facebook.com/kemmer2008
- username : kemmer2008
- bio : Nulla placeat aspernatur fuga amet.
- followers : 5150
- following : 2018
twitter:
- url : https://twitter.com/kemmer1974
- username : kemmer1974
- bio : Eum error autem quia. Voluptatem ut deleniti corporis eum. Aut est explicabo quia error debitis quia.
- followers : 5241
- following : 2701
linkedin:
- url : https://linkedin.com/in/orvalkemmer
- username : orvalkemmer
- bio : Aut ut quia accusamus quae.
- followers : 3407
- following : 440