Chlorine Trifluoride (ClF3) is an intriguing molecule with a unique Lewis dot structure that plays a pivotal role in the realm of chemistry. Grasping its structure offers a deeper understanding of molecular bonding and geometry, making it an essential concept for both students and professionals eager to unravel the complexities of molecular science.
Delving into the Lewis dot structure of ClF3 transcends mere academic curiosity; it serves as a practical application of theoretical chemistry with far-reaching implications across various industries. From materials science to environmental studies, the insights gained from understanding the Lewis dot structure of ClF3 reveal how molecules interact and form intricate compounds, impacting numerous fields of research and development.
In this extensive article, we will meticulously examine the nuances of the Lewis dot structure of ClF3. We will dissect its components, analyze its significance, and explore the reasons why this molecule captivates the interest of scientists and researchers. Join us as we embark on an enlightening journey into the world of ClF3 and uncover its secrets.
- Amc Grand Prairie
- Charlieheen Ashton Kutcher
- Buservice Greyhound
- Joe Biden Political Career
- Alice Braga Moraes
Understanding Chlorine Trifluoride (ClF3) and Its Importance
Chlorine Trifluoride (ClF3) is a highly reactive and toxic chemical compound, primarily utilized in the semiconductor industry for material etching and in the production of uranium hexafluoride for nuclear fuel processing. Deciphering its Lewis dot structure is paramount as it elucidates the molecule's behavior and reactivity, offering a foundation for comprehending its properties and applications.
ClF3 exhibits a T-shaped geometry, a result of its distinctive atomic arrangement. This structure significantly influences its chemical properties and interactions. By scrutinizing the Lewis dot structure, we uncover the reasons behind ClF3's potency as an oxidizing agent, enhancing our understanding of its role in various scientific and industrial contexts.
Grasping the Lewis Dot Structure
Definition and Fundamentals
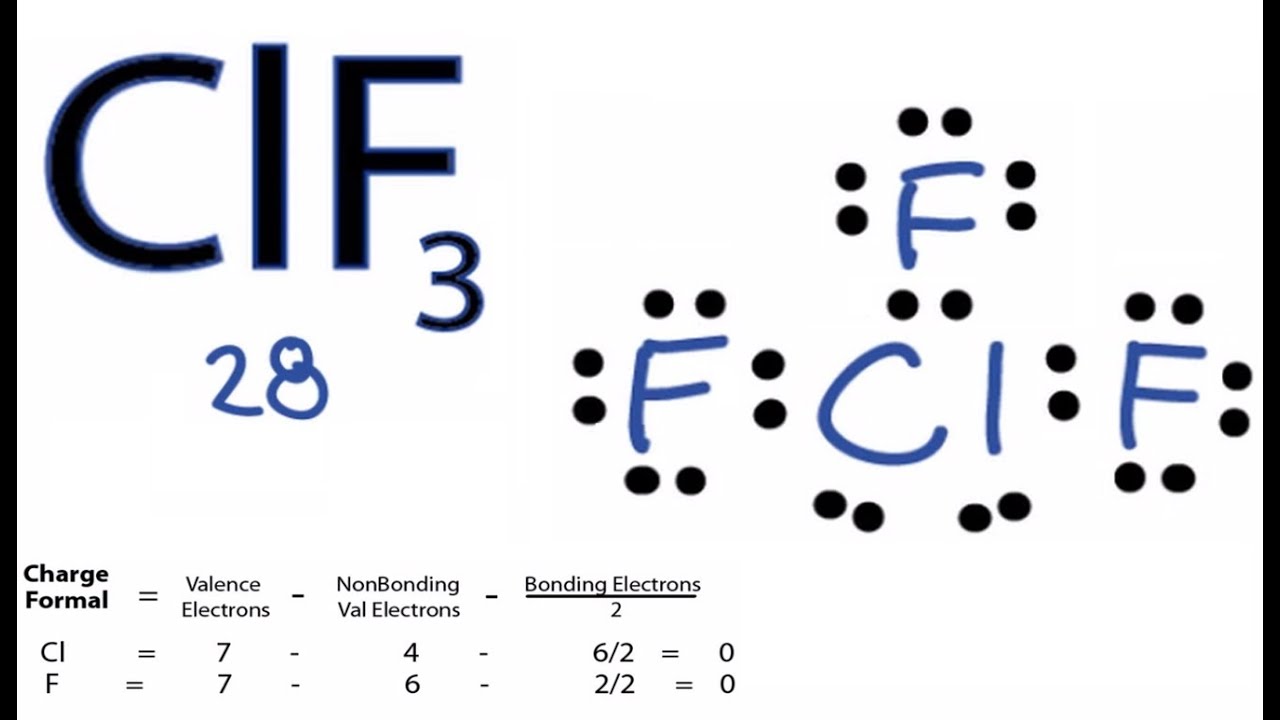
The Lewis dot structure is a visual representation that illustrates the valence electrons of atoms within a molecule. It aids in visualizing atomic bonding and the distribution of electrons, providing clarity on molecular interactions. For ClF3, the Lewis dot structure reveals how chlorine (Cl) bonds with three fluorine (F) atoms, featuring two lone pairs of electrons on the chlorine atom.
- Lolwarm Item Guide
- Brigitte Nielsen
- Donald Trump Children Names
- South Bend A Breaking News
- Modesto Family Court
- Chlorine contributes seven valence electrons.
- Each fluorine atom contributes seven valence electrons.
- In total, ClF3 consists of 28 valence electrons.
Guidelines for Drawing the Lewis Dot Structure of ClF3
Step 1: Identifying the Central Atom
In the case of ClF3, chlorine serves as the central atom due to its lower electronegativity compared to fluorine. This principle is foundational when constructing Lewis dot structures, positioning chlorine as the atom to which the fluorine atoms bond.
Step 2: Calculating Total Valence Electrons
To depict the Lewis dot structure, calculating the total number of valence electrons in the molecule is essential. For ClF3, this calculation unfolds as follows:
- Chlorine contributes seven electrons.
- Each fluorine atom contributes seven electrons, with three fluorine atoms present.
- Total = 7 + (7 x 3) = 28 valence electrons.
Step 3: Positioning Lone Pairs and Bonds
After identifying the central atom and determining the total valence electrons, the subsequent step involves placing lone pairs and bonds. Chlorine forms single bonds with each fluorine atom, with the remaining electrons allocated as lone pairs on the chlorine atom, ensuring the stability of the molecule.
Molecular Geometry of ClF3
T-shaped Configuration
The molecular geometry of ClF3 adopts a T-shaped form due to the presence of two lone pairs on the chlorine atom. These lone pairs repel the bonding pairs of electrons, resulting in a distinct geometry that sets ClF3 apart from molecules with similar formulas. The T-shaped structure is instrumental in comprehending the reactivity and properties of ClF3, influencing its interactions with other substances.
Electron Pair Geometry Versus Molecular Geometry
Distinguishing Between the Two
Although the electron pair geometry of ClF3 is trigonal bipyramidal, its molecular geometry manifests as T-shaped. This discrepancy arises from the influence of lone pairs on the chlorine atom, which alter the arrangement of bonding pairs. Recognizing this distinction is critical in forecasting molecular behavior, enabling scientists to predict and analyze chemical reactions with greater accuracy.
Characteristics of ClF3
Physical and Chemical Traits
ClF3 exists as a colorless gas at room temperature but transforms into a pale greenish-yellow liquid under pressure. Its highly reactive nature allows it to ignite combustible materials without external ignition sources. Key characteristics include:
- Molecular Weight: 92.45 g/mol
- Boiling Point: 11.78°C
- Melting Point: -76.3°C
Utilizations of ClF3
Industrial Applications
Owing to its unique properties, ClF3 finds extensive applications in several industries. Notable uses encompass:
- Semiconductor Manufacturing: Employed for etching silicon wafers with precision.
- Nuclear Industry: Utilized in the production of uranium hexafluoride, crucial for nuclear fuel processing.
- Propellants: Leveraged in rocket propellants due to its exceptional oxidizing capabilities.
Safety Measures
Managing ClF3 Safely
Given its highly reactive and toxic nature, handling ClF3 necessitates meticulous safety measures. Essential precautions include:
- Utilizing appropriate personal protective equipment (PPE) to safeguard against exposure.
- Operating in well-ventilated environments or fume hoods to minimize inhalation risks.
- Adhering to stringent safety protocols during storage, transportation, and disposal to ensure secure handling.
Environmental Considerations
Addressing the Environmental Impact
ClF3 poses significant environmental challenges due to its reactivity and toxicity. It reacts violently with water and organic materials, releasing harmful gases that can adversely affect ecosystems. Proper disposal and containment practices are imperative to mitigate its environmental impact and safeguard ecological health.
Summary
The Lewis dot structure of ClF3 is an enthralling subject that bridges theoretical chemistry with practical applications. By comprehending its structure, we gain valuable insights into its reactivity, properties, and diverse uses. ClF3 is a powerful molecule utilized across various industries, yet its handling demands caution owing to its toxic and reactive nature.
We invite readers to further explore the realm of molecular chemistry and expand their knowledge. Share your thoughts and inquiries in the comments section, and explore other articles on our platform to deepen your understanding of the captivating world of chemistry.
Table of Contents
- Understanding Chlorine Trifluoride (ClF3) and Its Importance
- Grasping the Lewis Dot Structure
- Guidelines for Drawing the Lewis Dot Structure of ClF3
- Molecular Geometry of ClF3
- Electron Pair Geometry Versus Molecular Geometry
- Characteristics of ClF3
- Utilizations of ClF3
- Safety Measures
- Environmental Considerations
- Summary
References
The content of this article is supported by credible sources, including:


Detail Author:
- Name : Bridie Vandervort II
- Username : richard.lind
- Email : shanahan.susanna@gmail.com
- Birthdate : 1970-12-02
- Address : 77820 Tina Cape Suite 128 Brodyburgh, PA 41990
- Phone : (925) 976-4317
- Company : Maggio-Bailey
- Job : Occupational Therapist Assistant
- Bio : Minus natus dicta vel molestiae sint praesentium. Qui rerum perspiciatis atque dolore excepturi. Pariatur accusantium sit neque hic et itaque.
Socials
tiktok:
- url : https://tiktok.com/@upton2024
- username : upton2024
- bio : Corporis aspernatur ab illum et qui aut est. Quo debitis labore voluptatem.
- followers : 4422
- following : 492
instagram:
- url : https://instagram.com/upton1997
- username : upton1997
- bio : Distinctio ut doloremque tempore. Natus ipsam et iste assumenda officiis minus quia repudiandae.
- followers : 6092
- following : 1856
twitter:
- url : https://twitter.com/vincenzaupton
- username : vincenzaupton
- bio : Dolorum at quisquam quaerat quam ut temporibus. Incidunt delectus placeat error adipisci aliquam non. Officiis sint et ea ea.
- followers : 5551
- following : 2303
linkedin:
- url : https://linkedin.com/in/uptonv
- username : uptonv
- bio : Laboriosam in explicabo quia velit tempore a.
- followers : 4267
- following : 1654