Mastering the Lewis structure of chlorine trifluoride (ClF₃) is indispensable for anyone diving into the world of chemistry, particularly when studying molecular geometry and chemical bonding. This compound, renowned for its distinctive T-shaped molecular structure, plays a pivotal role in comprehending the behavior of interhalogen compounds. By analyzing its Lewis structure, chemists can anticipate the compound's reactivity, stability, and interactions with other substances, unlocking deeper insights into its chemical properties.
Chlorine trifluoride is an intriguing molecule due to its capacity to form robust bonds while preserving a unique molecular shape. In this comprehensive article, we will explore the complexities of ClF₃'s Lewis structure, examining its formation, characteristics, and implications in chemical reactions. Whether you're a student, researcher, or simply fascinated by molecular structures, this guide will offer valuable perspectives on ClF₃'s properties.
Our in-depth exploration will encompass critical topics such as electron distribution, bond angles, and molecular geometry. Additionally, we will delve into the significance of the Lewis structure in predicting the behavior of chlorine trifluoride across various chemical environments. Let's embark on this journey by examining the foundational aspects of this remarkable molecule.
- Power Outage Entergy
- Temperature For Medium Rareteak
- Return Policy Forteam
- Charlieheen Ashton Kutcher
- Tom And Jerry 2020 Cast
Table of Contents
- Introduction to Lewis Structure
- Basics of Lewis Structures
- Overview of Chlorine Trifluoride
- Electron Distribution in ClF₃
- Molecular Geometry
- Bond Angles
- Resonance Structures
- Applications of ClF₃
- Safety Considerations
- Conclusion
Understanding the Importance of Lewis Structures
The Lewis structure serves as a cornerstone in chemistry, offering a visual representation of the arrangement of valence electrons within a molecule. By mapping these electrons, chemists can predict the molecule's shape, polarity, and reactivity. For chlorine trifluoride (ClF₃), the Lewis structure provides profound insights into its unique T-shaped geometry and the distribution of electrons around the central chlorine atom.
Why Lewis Structures Are Essential
Lewis structures are indispensable tools for unraveling the mysteries of molecular bonding. They enable chemists to visualize how atoms share electrons and achieve stable configurations. In the case of ClF₃, the structure unveils the presence of lone pairs and bonded pairs, which profoundly influence the molecule's geometry and overall properties.
The Fundamentals of Lewis Structures
A Lewis structure is a two-dimensional diagram that illustrates how valence electrons are allocated among the atoms in a molecule. To construct a Lewis structure, chemists adhere to a set of guidelines:
- Calculate the total number of valence electrons in the molecule.
- Identify the central atom and arrange the surrounding atoms accordingly.
- Distribute electrons to ensure each atom satisfies the octet rule.
- Address any formal charges if necessary.
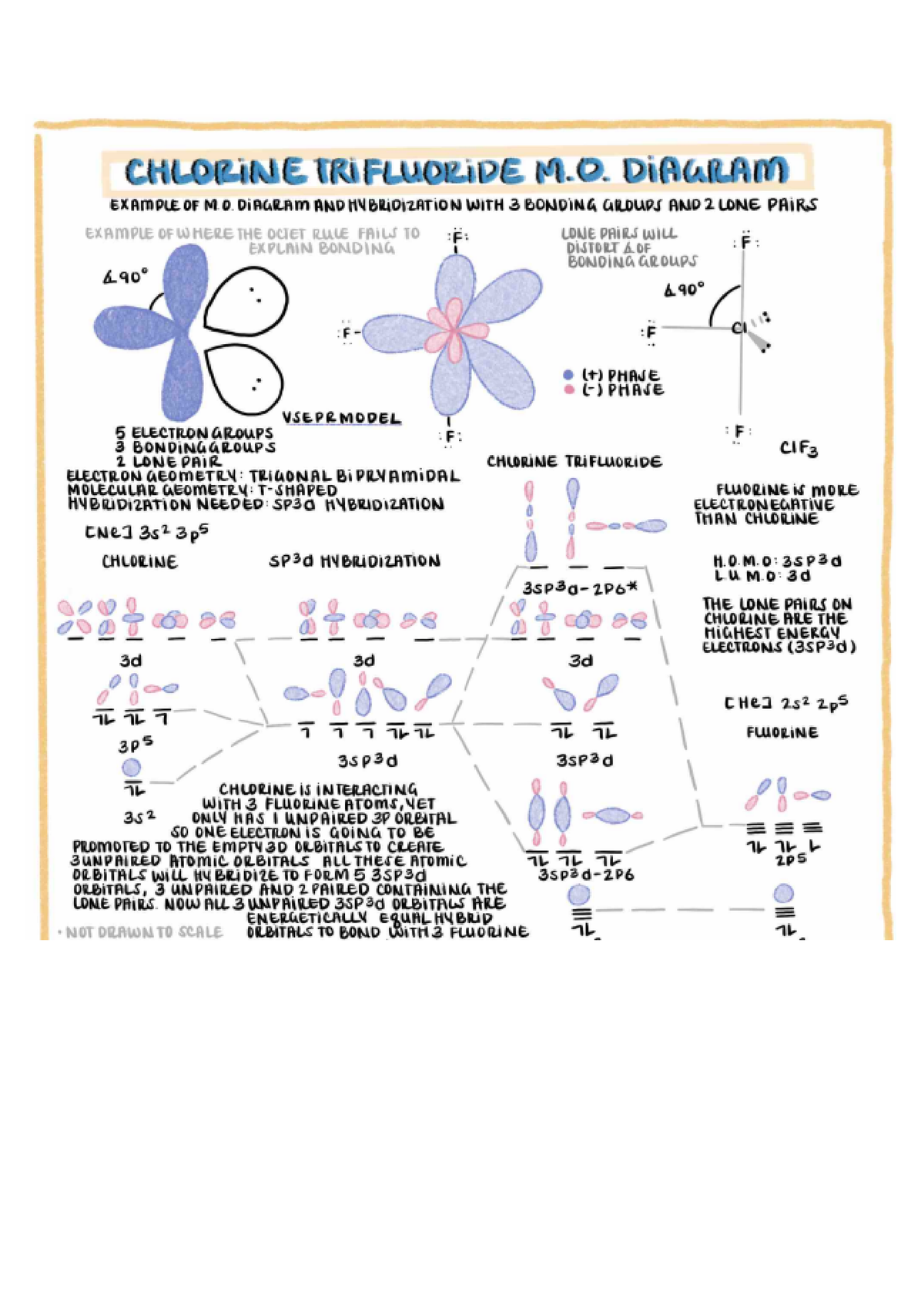
In the case of ClF₃, the central chlorine atom forms three single bonds with fluorine atoms, leaving two lone pairs of electrons on the chlorine atom, contributing to its unique geometry.
A Closer Look at Chlorine Trifluoride
Chlorine trifluoride (ClF₃) is a highly reactive interhalogen compound composed of one chlorine atom and three fluorine atoms. First synthesized in 1936, it has since been utilized in a variety of industrial applications, including rocket propellants and chemical manufacturing processes.
Key Characteristics of ClF₃
ClF₃ exists as a colorless gas at room temperature but can transform into a pale green liquid under pressure. It is highly toxic and corrosive, necessitating meticulous handling in both laboratory and industrial settings. The molecule's reactivity is deeply rooted in its electron distribution and molecular geometry, which we will dissect thoroughly.
Analyzing Electron Distribution in ClF₃
Within the Lewis structure of ClF₃, the central chlorine atom contributes seven valence electrons, while each fluorine atom contributes seven valence electrons. This results in a total of 28 valence electrons for the molecule. These electrons are allocated as follows:
- Three single bonds between chlorine and fluorine atoms, utilizing six electrons.
- Two lone pairs on the chlorine atom, accounting for four electrons.
- Remaining electrons fulfill the octet rule for each fluorine atom.
This distribution leads to the formation of a T-shaped molecular geometry, as the lone pairs on chlorine repel the bonding pairs, positioning the fluorine atoms to minimize repulsion.
Exploring the Molecular Geometry of ClF₃
The molecular geometry of ClF₃ is determined by the Valence Shell Electron Pair Repulsion (VSEPR) theory, which forecasts the arrangement of electron pairs around the central atom. In ClF₃, the two lone pairs on chlorine generate significant repulsion, resulting in a T-shaped geometry instead of a trigonal bipyramidal shape.
Factors Influencing the Geometry
Several elements contribute to ClF₃'s unique geometry:
- The existence of lone pairs on the central atom.
- The electronegativity difference between chlorine and fluorine.
- The minimization of electron pair repulsion as per VSEPR theory.
Grasping these factors is vital for forecasting the behavior of ClF₃ in chemical reactions and its interactions with other substances.
Investigating Bond Angles in ClF₃
The bond angles in ClF₃ are slightly less than 90° due to the repulsion between the lone pairs and bonding pairs of electrons. This deviation from the ideal geometry arises from the stronger lone pair-bond pair repulsion compared to bond pair-bond pair repulsion. Experimental data indicates bond angles of approximately 87.5° between the axial and equatorial fluorine atoms.
The Relevance of Bond Angles
The bond angles in ClF₃ have significant implications for its chemical properties:
- They define the molecule's overall shape and polarity.
- They affect the molecule's reactivity with other substances.
- They provide insights into the stability of the molecule under varying conditions.
By comprehending these angles, chemists can more accurately predict ClF₃'s behavior in diverse chemical settings.
Delving into Resonance Structures
Although ClF₃ does not exhibit classical resonance structures, the distribution of electrons around the central chlorine atom can be scrutinized through formal charge considerations. The lone pairs on chlorine enhance the molecule's stability, ensuring that the bonding configuration aligns with the octet rule.
The Role of Stability
The absence of resonance structures in ClF₃ underscores the importance of electron distribution in maintaining molecular stability. The lone pairs on chlorine play a critical role in reducing repulsion and ensuring the molecule's overall stability. This stability is crucial for ClF₃'s applications in various chemical processes.
Applications of ClF₃ in Industry
Chlorine trifluoride finds utility in numerous industries due to its distinctive chemical properties:
- Rocket Propellants: ClF₃ serves as an oxidizer in rocket fuel, leveraging its high reactivity to release substantial amounts of energy.
- Chemical Manufacturing: It functions as a fluorinating agent in the production of various fluorinated compounds.
- Material Processing: ClF₃ is employed in the etching of materials, especially in semiconductor manufacturing.
Despite its practicality, ClF₃ demands cautious handling due to its toxicity and corrosiveness.
Essential Safety Precautions
Managing ClF₃ necessitates strict compliance with safety protocols given its hazardous nature. The compound is highly reactive, toxic, and corrosive, posing considerable risks to human health and the environment. Safety measures include:
- Utilizing personal protective equipment (PPE) such as gloves, goggles, and respirators.
- Operating in well-ventilated areas or fume hoods.
- Storing ClF₃ in compatible containers away from moisture and other reactive substances.
By adhering to these precautions, researchers and industrial workers can handle ClF₃ safely while minimizing risks.
Final Thoughts on Chlorine Trifluoride
The Lewis structure of chlorine trifluoride offers invaluable insights into its molecular geometry, electron distribution, and chemical properties. By understanding the T-shaped geometry and bond angles of ClF₃, chemists can anticipate its behavior in various applications, ranging from rocket propellants to semiconductor manufacturing. However, due to its toxicity and reactivity, ClF₃ must be handled with extreme care.
We encourage readers to explore additional resources on molecular structures and chemical bonding to enhance their comprehension of this fascinating compound. Please share your thoughts and questions in the comments section below, and consider sharing this article with others who might find it enlightening. For more chemistry-related content, delve into our other articles and resources.
References:
- Brown, T. L., LeMay, H. E., Bursten, B. E., & Murphy, C. J. (2014). Chemistry: The Central Science. Pearson Education.
- Housecroft, C. E., & Sharpe, A. G. (2012). Inorganic Chemistry. Pearson Education.
- Moore, J. W., Stanitski, C. L., & Jurs, P. C. (2009). Chemistry: The Molecular Science. Cengage Learning.

Detail Author:
- Name : Elisha Reichert
- Username : nella.swift
- Email : cooper87@rowe.com
- Birthdate : 1993-12-12
- Address : 852 Botsford Highway West Hank, WI 34492-5991
- Phone : 816-383-2086
- Company : Crist, Fisher and Willms
- Job : Insulation Installer
- Bio : Cum minima ipsum consequatur quas dolorem totam. Omnis minus laborum libero mollitia. Quia dignissimos sunt et et suscipit suscipit. Nam ut earum ullam soluta.
Socials
tiktok:
- url : https://tiktok.com/@leann6705
- username : leann6705
- bio : Eaque non ipsum illum molestias dolor sapiente et.
- followers : 6702
- following : 1299
facebook:
- url : https://facebook.com/thiel1977
- username : thiel1977
- bio : Ut velit distinctio eos quidem reprehenderit.
- followers : 1350
- following : 2983
linkedin:
- url : https://linkedin.com/in/leannthiel
- username : leannthiel
- bio : Aliquid et maiores voluptatum.
- followers : 1483
- following : 1948
instagram:
- url : https://instagram.com/leann505
- username : leann505
- bio : Modi est excepturi sapiente iusto. Illum et aliquid aliquid. Ut vitae optio ut ut.
- followers : 5278
- following : 1547
twitter:
- url : https://twitter.com/leannthiel
- username : leannthiel
- bio : Perferendis quis reiciendis mollitia. Quisquam nihil temporibus commodi molestias excepturi. Quae dolorem exercitationem id vel dolor quis commodi.
- followers : 6753
- following : 611