Understanding the molecular geometry of carbon dioxide (CO2) provides a deeper insight into the arrangement of atoms in this critical molecule. CO2 plays an indispensable role in our atmosphere, influencing the greenhouse effect and regulating global climate patterns. Delving into its molecular geometry helps us comprehend its behavior and interactions with other substances, making it a cornerstone of both chemistry and environmental science.
Molecular geometry serves as the foundation of chemistry, offering profound insights into how molecules function and behave. CO2, a seemingly simple molecule composed of one carbon atom and two oxygen atoms, holds immense significance due to its linear geometry and symmetrical structure. This arrangement makes it an ideal subject for studying molecular behavior, providing valuable information for scientific research and industrial applications.
Through this article, we aim to deliver a comprehensive analysis of CO2 molecular geometry, exploring its structure, properties, and implications in chemistry and environmental science. Whether you're a student, researcher, or simply curious about the world of molecules, this guide will enhance your understanding of CO2 and its role in shaping our environment.
- 60 Minutes What Is On Tonight
- How Do I Order Checks From Chase
- Charlieheen Ashton Kutcher
- Return Policy Forteam
- Road Closures In Kansas
Table of Contents
- Introduction to CO2 Molecular Geometry
- CO2 Structure and Bonding
- VSEPR Theory and CO2
- Linear Geometry of CO2
- Symmetry and Properties
- Bond Angle in CO2
- Environmental Impact
- Applications in Industry
- Frequently Asked Questions
- Conclusion
Understanding the Spatial Arrangement of CO2
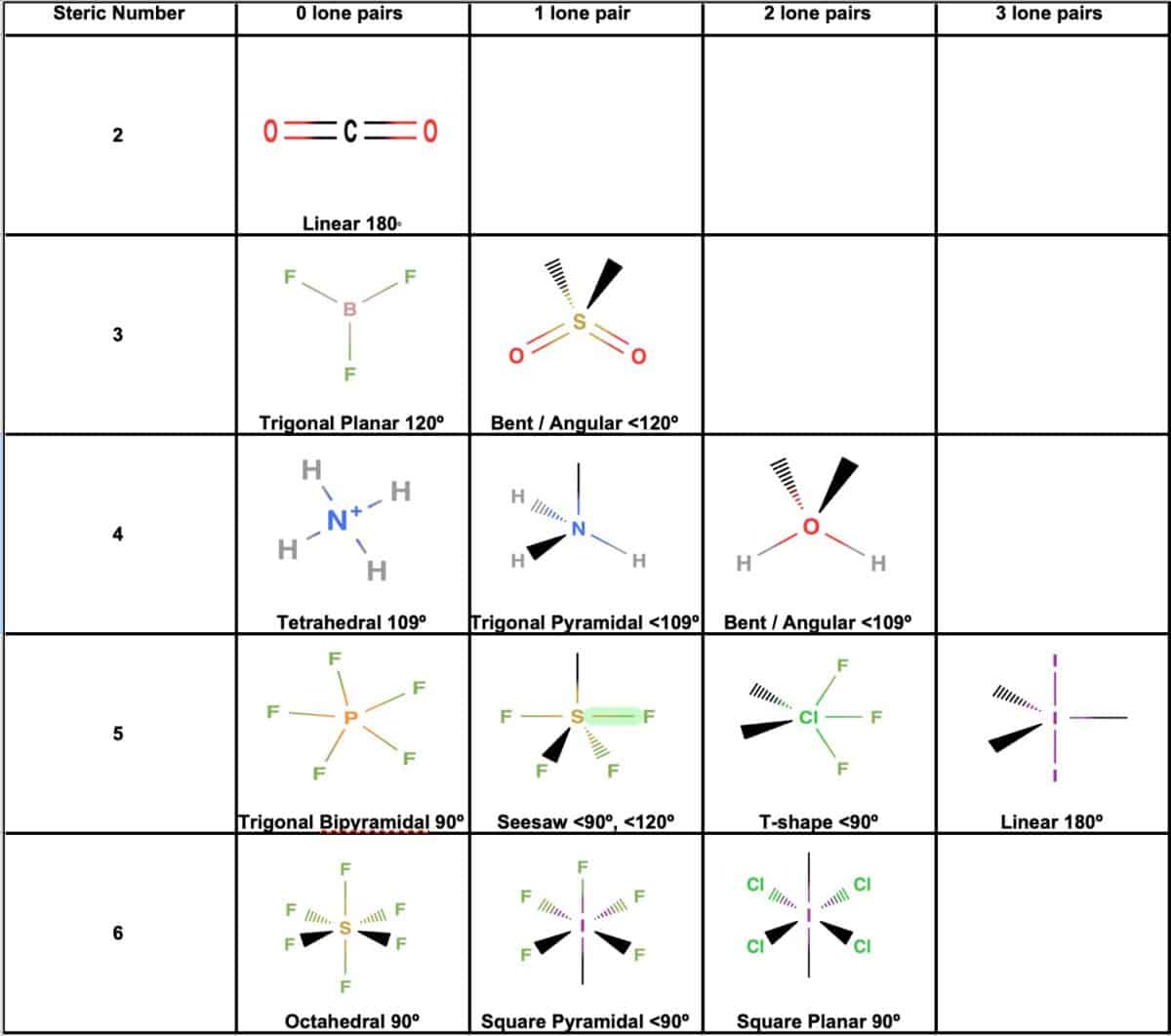
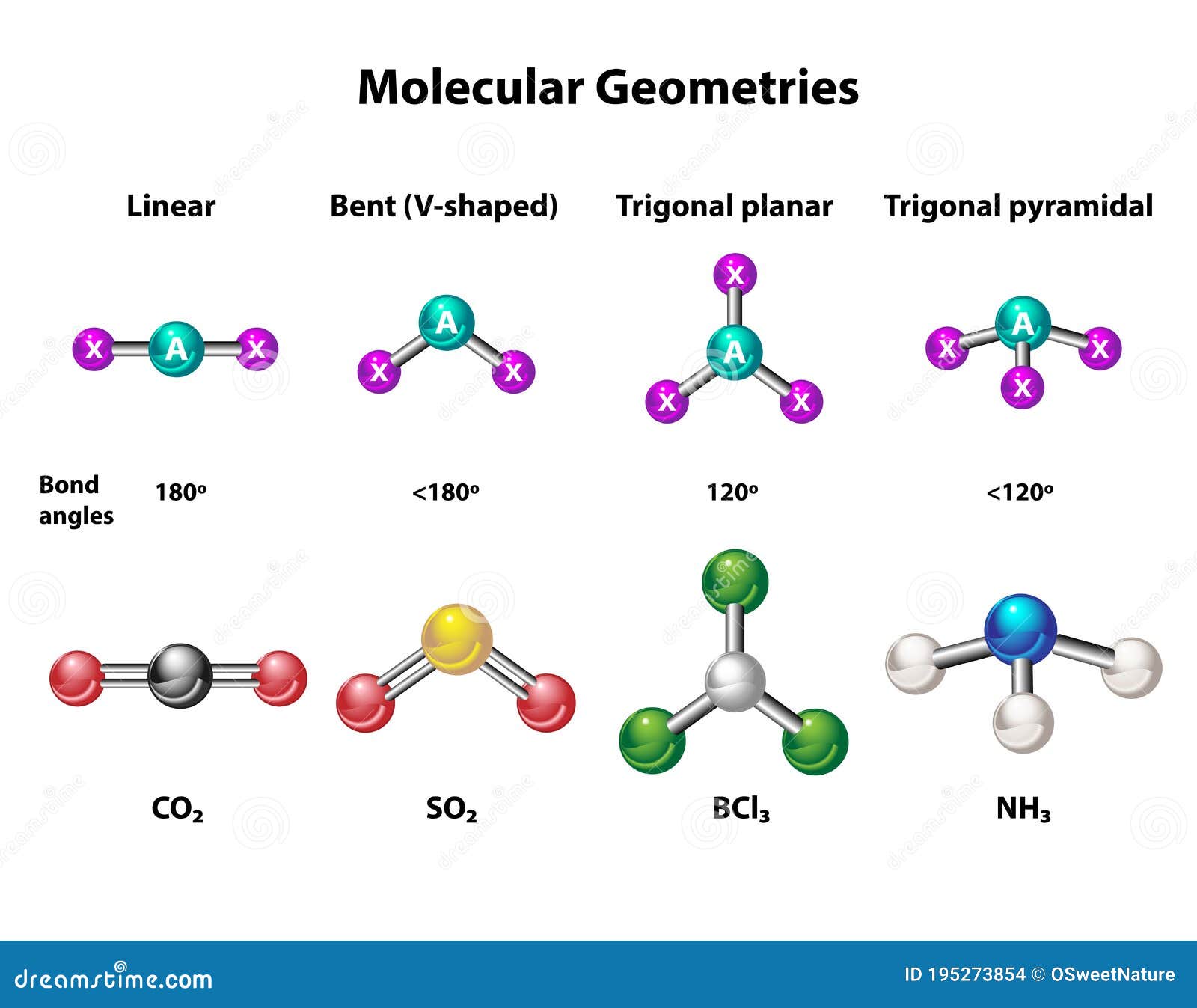
The study of CO2 molecular geometry focuses on the spatial arrangement of atoms within the carbon dioxide molecule. Carbon dioxide is a linear molecule, with its atoms aligned in a straight line. This alignment is governed by the Valence Shell Electron Pair Repulsion (VSEPR) theory, which predicts molecular shapes based on the repulsion between electron pairs. The VSEPR theory explains why CO2 adopts a linear geometry.
The molecular geometry of CO2 is linear, characterized by a bond angle of 180 degrees. The molecule's symmetry contributes to its nonpolar nature, making it an excellent solvent for various substances. Furthermore, the geometric structure of CO2 significantly influences its reactivity and interactions with other molecules, playing a crucial role in both natural and industrial processes.
This section delves into the fundamental principles behind CO2 molecular geometry and highlights its importance in chemistry and environmental science.
- Premier Row
- Shopritetore Locator Pa
- Caesars Property Map
- Donald Trump Children Names
- Quality Inn Hotel Ocean City Md
CO2 Structure and Bonding: A Closer Look
Atomic Composition
CO2 is composed of one carbon atom and two oxygen atoms. The carbon atom forms double bonds with each oxygen atom, creating a stable and symmetric molecule. This double bonding is essential in determining the molecule's geometry and properties, ensuring its stability and resistance to chemical reactions.
Bonding Mechanism
The bonding in CO2 involves the sharing of electrons between the carbon and oxygen atoms. Each oxygen atom shares two electrons with the carbon atom, forming a double bond. This arrangement ensures that all atoms achieve a stable electron configuration, similar to that of noble gases. Understanding the bonding mechanism in CO2 provides valuable insights into its stability, reactivity, and interactions with other substances.
Applying VSEPR Theory to CO2
Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of molecules based on the repulsion between electron pairs. In the case of CO2, the VSEPR theory predicts a linear geometry due to the absence of lone pairs on the central carbon atom. The theory considers the number of electron pairs surrounding the central atom and their spatial arrangement. For CO2, the central carbon atom has two bonding pairs and no lone pairs, resulting in a linear shape with a bond angle of 180 degrees.
This section explores the application of VSEPR theory to CO2 and its implications for molecular geometry, offering a deeper understanding of the molecule's structure and properties.
Characteristics and Implications of CO2's Linear Geometry
Characteristics of Linear Geometry
The linear geometry of CO2 is defined by its straight-line arrangement of atoms and a bond angle of 180 degrees. This geometry arises from the symmetric distribution of electron pairs around the central carbon atom, ensuring minimal repulsion and maximum stability. The linear arrangement of atoms in CO2 contributes to its unique physical and chemical properties, making it an ideal molecule for various applications.
Implications of Linear Geometry
The linear geometry of CO2 has significant implications for its physical and chemical properties. It contributes to the molecule's nonpolar nature, making it insoluble in water but highly soluble in organic solvents. Additionally, the linear shape enhances CO2's stability and resistance to chemical reactions, making it an essential component in industrial processes and environmental systems.
This section examines the characteristics and implications of the linear geometry of CO2, highlighting its importance in both scientific research and practical applications.
Symmetry and Properties: The Role of CO2
Symmetry plays a pivotal role in determining the properties of CO2. The molecule's symmetric structure results in a balanced distribution of charge, making it nonpolar. This nonpolar nature significantly influences its interactions with other substances and its behavior in various environments. CO2's symmetry and properties make it a versatile molecule with applications in chemistry, environmental science, and industry.
Key properties of CO2 include:
- High stability, ensuring its longevity in various conditions
- Nonpolar nature, allowing it to interact effectively with nonpolar substances
- Excellent solvent properties, making it suitable for dissolving a wide range of materials
- Significant contribution to the greenhouse effect, influencing global climate patterns
This section explores the symmetry of CO2 and its impact on the molecule's properties, offering a comprehensive understanding of its role in shaping our world.
The Bond Angle in CO2: A Fundamental Aspect
Understanding Bond Angles
The bond angle in CO2 is precisely 180 degrees, a direct result of its linear geometry. This bond angle arises from the symmetric arrangement of electron pairs around the central carbon atom, minimizing repulsion and maximizing stability. The linear arrangement ensures optimal distribution of electron pairs, contributing to the molecule's stability and symmetry.
Significance of Bond Angle
The bond angle in CO2 is crucial as it determines the molecule's shape and influences its interactions with other molecules. A bond angle of 180 degrees ensures optimal stability and symmetry, contributing to the molecule's nonpolar nature and enhancing its utility in various applications. Understanding the bond angle in CO2 provides valuable insights into its molecular geometry and behavior.
CO2's Role in Environmental Science
CO2 is a major contributor to the greenhouse effect, playing a critical role in regulating Earth's climate. Its molecular geometry and properties make it an effective absorber of infrared radiation, trapping heat in the atmosphere and contributing to global warming. According to the Intergovernmental Panel on Climate Change (IPCC), CO2 levels have increased significantly over the past century due to human activities such as fossil fuel combustion and deforestation. This rise in CO2 levels has profound implications for global climate patterns and ecosystems, necessitating urgent action to mitigate its effects.
Industrial Applications of CO2
CO2 finds extensive applications in various industries due to its unique properties. Its linear geometry and nonpolar nature make it an ideal candidate for these applications, while its stability and solubility properties further enhance its utility in industrial processes. Some of its industrial uses include:
- Carbonation in beverages, providing fizz and enhancing taste
- Fire extinguishers, leveraging its non-flammable and non-toxic properties
- Refrigerants, utilizing its ability to cool substances effectively
- Supercritical CO2 extraction, offering a sustainable method for extracting compounds
- Enhanced oil recovery, improving oil extraction efficiency
This section highlights the diverse applications of CO2 in industry, emphasizing its importance in modern technology and sustainable development.
Frequently Asked Questions About CO2 Molecular Geometry
What is the molecular geometry of CO2?
The molecular geometry of CO2 is linear, characterized by a bond angle of 180 degrees. This geometry arises from the symmetric arrangement of electron pairs around the central carbon atom, ensuring minimal repulsion and maximum stability.
Why is CO2 nonpolar?
CO2 is nonpolar due to its symmetric geometry, which results in a balanced distribution of charge. The equal sharing of electrons between carbon and oxygen atoms ensures that the molecule has no net dipole moment, making it an excellent solvent for nonpolar substances.
How does CO2 contribute to the greenhouse effect?
CO2 absorbs infrared radiation, trapping heat in the Earth's atmosphere and contributing to the greenhouse effect. Its molecular geometry and properties make it an effective absorber of heat, influencing global climate patterns and necessitating efforts to reduce its emissions.
This section addresses common questions about CO2 molecular geometry and its implications, providing clarity and enhancing understanding.
Conclusion: The Importance of CO2 Molecular Geometry
In conclusion, CO2 molecular geometry is a vital subject that offers profound insights into the structure and behavior of this essential molecule. The linear geometry and symmetric structure of CO2 contribute to its stability, nonpolar nature, and diverse applications in industry and environmental science. By understanding the science behind CO2, we can better address the challenges posed by climate change and harness the molecule's potential for sustainable development.
We encourage readers to explore further resources and studies on CO2 molecular geometry. By deepening your understanding of this molecule, you can contribute to addressing global challenges and advancing scientific knowledge. Please share your thoughts and questions in the comments section below, and feel free to share this article with others who may find it informative. Explore our other articles for more in-depth knowledge on related topics.

Detail Author:
- Name : Sheila O'Conner
- Username : fkozey
- Email : jhyatt@senger.com
- Birthdate : 2006-10-21
- Address : 170 Wilber Courts New Thaddeus, IL 00737
- Phone : 640-581-5921
- Company : Wyman and Sons
- Job : Order Filler OR Stock Clerk
- Bio : Necessitatibus sed reprehenderit dolor tempora enim dolorem enim. Veniam aut voluptas qui error accusamus qui ullam. Ab quas rem ad perspiciatis beatae aut vel.
Socials
instagram:
- url : https://instagram.com/cschumm
- username : cschumm
- bio : Est dolor et ex et vel. Commodi voluptatibus labore autem fuga accusamus.
- followers : 3272
- following : 1752
tiktok:
- url : https://tiktok.com/@chris.schumm
- username : chris.schumm
- bio : Corporis adipisci voluptatem et dolorem vero tenetur est.
- followers : 1932
- following : 847
facebook:
- url : https://facebook.com/chris1783
- username : chris1783
- bio : Voluptas sed at et. Error ipsam atque ad qui. Quam a et quisquam consequatur.
- followers : 6827
- following : 1941
twitter:
- url : https://twitter.com/chris6000
- username : chris6000
- bio : Optio excepturi atque nemo dolorem et adipisci accusantium. Non sed repellendus explicabo rerum ipsum.
- followers : 4852
- following : 241
linkedin:
- url : https://linkedin.com/in/chris_xx
- username : chris_xx
- bio : Voluptatem vel ut et.
- followers : 6680
- following : 2991